Investigate insights into three trials which utilized DAVA’s Physician Focused Trial Acceleration and Clinical Trial Education Workshops
Non-Small Cell Lung Cancer
Accrual Workshops Results for PD-L1 NSCLC Trials
Tumor type: Front-line or Relapsed/Refractory Non-small Cell Lung Cancer (NSCLC)
Phase: 6 Phase III trials
Goal of DAVA’s involvement: Employ Accrual Workshops to motivate increased enrollment across multiple trials
Recruitment tactic: Organize a series of Accrual Workshops around the world, both standalone workshops and workshops in conjunction with investigator meetings
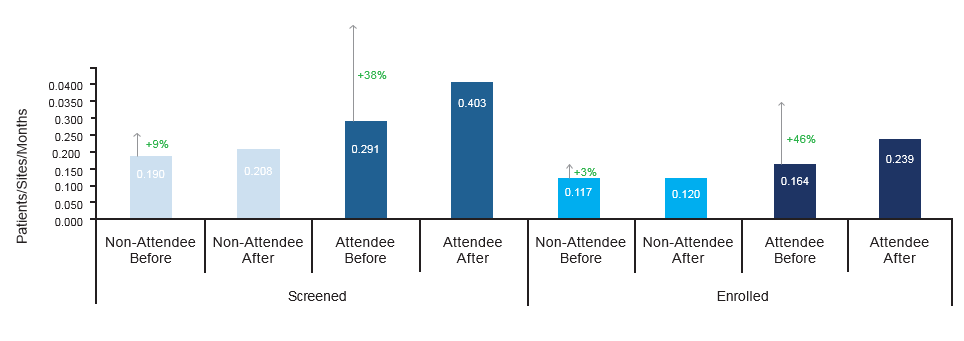
Open Sites attending a workshop: 177 Open Sites not attending a workshop: 463 Workshops evaluated: 23
Results: Both attending and non-attending sites’ screening and enrollment rates were evaluated over a 60-day period before and after each accrual workshop. On average, sites sending representatives to DAVA’s Accrual Workshops demonstrated a 38% increase in screening rate and a 46% increase in enrollment rate in the 60 days after attending a workshop versus the 60 days prior to attending the workshop. Sites that did not attend a workshop evaluated over the same time periods demonstrated, on average, only a 9% increase in screening rate and a 3% increase in enrollment rate. The marked increase in screening and enrollment rates for attending sites is indicative of attendees’ augmented abilities to identify eligible patients to screen for the trial and is a common trend for sites that attend DAVA’s Accrual Workshops.
Comparison of Screening and Enrollment Rates for Sites According to Attendance at DAVA Accrual Workshops
Hematologic Malignancies
Tumor type: Hematologic malignancies post-autologous HSCT
Phase: III
Enrollment Goal: 1,204
Sites: 65-70 active autologous HSCT sites (US only) – Only 112 US adult transplant sites are accredited by the National Marrow Donor Program
Goal of DAVA’s involvement: Use DAVA’s expertise and professional connections to identify accredited transplant sites willing to participate on the trial, including sites that had been previously unresponsive to the sponsor or who had previously declined to participate.
Site recommendation tactics: Review the sponsor’s site list and identify potential new sites, identify the correct investigator contact at each site, and make direct MD-to-MD contacts with the site to discuss the trial and encourage interest in participating.
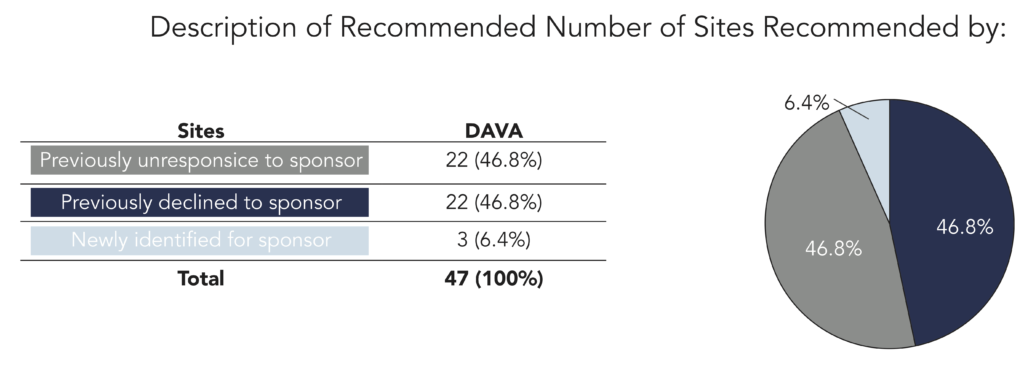
Results: Despite the limited pool of sites and the sponsors’ previous attempts to gain sites’ commitment, DAVA obtained positive responses from 22 previously unresponsive sites, converted 22 negative responses to positive responses, and identified 3 previously uncontacted sites willing to participate on the trial. In all, DAVA recommended 47 of the 67 total sites ultimately selected for the trial.
Soft Tissue Sarcoma
Tumor type: Advanced Soft Tissue Sarcoma
Phase: III
Enrollment goal: 212 patients (US only)
Sites: 74 (US only)
Goal of DAVA’s involvement: Increase enrollment rate of 74 US sites during the 15-month study engagement period. DAVA’s enrollment target was to enroll 212 patients in the US by the end of study.
Recruitment tactic: Physician-Focused Patient Recruitment, initiated 9 months after trial opened.
Results: By deploying DAVA medical oncologists and the clinical trial team to engage sites in patient identification and recruitment strategies, DAVA increased the total US enrollment to 244 patients, which accounted for 56% of the global enrollment, and increased the enrollment rate from 0.118 to 0.216 patients/site/month. Initially, DAVA was only given responsibility for the low-enrolling sites. However, after the interim analysis presented below was shared with the sponsor, where it was shown that formerly high-preforming sites not supported by DAVA had suffered a 32% decrease in enrollment rate, while previously under-performing sites.
DAVA Supported Sites
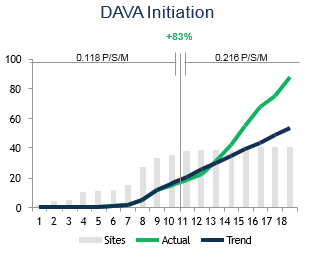
Non-DAVA Supported Sites
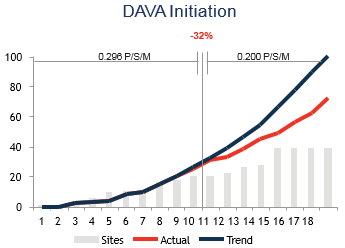
Months